Is NH3 Polar or Nonpolar? A Comprehensive Guide to Understand NH3 Molecule
NH3 is a chemical formula for Ammonia. Ammonia is a colorless, pungent-smelling gas, which is widely used in various industrial processes. In this article, we will discuss the polarity of NH3, whether it is polar or nonpolar, and how to determine it.
Understanding NH3 Molecule
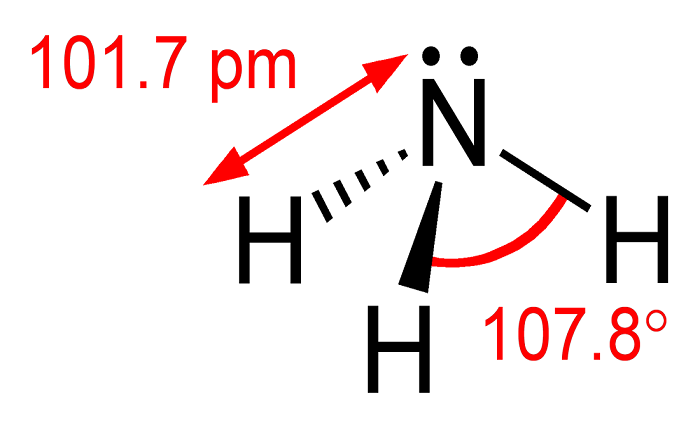
To understand whether NH3 is polar or nonpolar, we need to understand its molecular structure. The NH3 molecule consists of one nitrogen atom and three hydrogen atoms. The nitrogen atom is at the center of the molecule, and the three hydrogen atoms are attached to it.
Electronegativity and Polarity
Electronegativity is a measure of an atom’s ability to attract electrons towards itself. In NH3, nitrogen has a higher electronegativity value (3.04) than hydrogen (2.2). Due to the difference in electronegativity values, the nitrogen atom attracts the shared electrons towards itself, creating a partial negative charge (δ-) on the nitrogen atom and a partial positive charge (δ+) on the hydrogen atoms.
Determining Polarity Using Dipole Moment
The dipole moment is a measure of the magnitude of the separation of positive and negative charges in a molecule. In NH3, the dipole moment is 1.47 D, indicating that the NH3 molecule is polar.
Polarity and Physical Properties of NH3
Polarity affects the physical properties of molecules. NH3 is a polar molecule, which means it has dipole-dipole interactions between molecules. These interactions result in a higher boiling point, melting point, and surface tension of NH3 compared to nonpolar molecules.
Applications of NH3
Ammonia is used in various industrial processes, including fertilizers, refrigeration, and cleaning agents. The polarity of NH3 plays a crucial role in its applications, as it determines its solubility in polar solvents and its ability to form hydrogen bonds.
NH3 and Health Hazards
Ammonia is toxic to humans and can cause respiratory problems, eye irritation, and skin burns. It is essential to handle NH3 with care and follow safety guidelines while using it.
Conclusion
In conclusion, NH3 is a polar molecule due to the difference in electronegativity values between nitrogen and hydrogen atoms. The polarity of NH3 influences its physical properties and applications. It is crucial to handle NH3 with care and follow safety guidelines while using it.
FAQs
- Why is NH3 polar?
- NH3 is polar due to the difference in electronegativity values between nitrogen and hydrogen atoms.
- What is the dipole moment of NH3?
- The dipole moment of NH3 is 1.47 D.
- What are the physical properties of NH3?
- NH3 has a higher boiling point, melting point, and surface tension due to its polar nature.
- What are the applications of NH3?
- NH3 is used in fertilizers, refrigeration, and cleaning agents.
- Is NH3 toxic to humans?
- Yes, NH3 is toxic to humans and can cause respiratory problems, eye irritation, and skin burns.